
© Blauplanet.com
Other sections:




Cyclic GMP
Regulation
Created by Dr. Luis Agulló (last update on 12-6-2007)
Regulation of the Pathway by Protein Kinase G
The cyclic GMP pathway is regulated at the level of the different enzymes implicated. This regulation is complex and in the case of certain key enzymes is treated in specific pages of this web site (see for example the regulation of nitric oxide synthase or NOS in the page eNOS REGULATION). However, some factors can be considered general regulators of the pathway. This is the case, for example, of calcium and of protein kinase G (PKG).
Calcium
This cation increase the activity of calcium-dependent nitric oxide synthases (NOS-I and NOS-III), a key step in the synthesis of cyclic GMP by soluble guanylyl cyclase. On the other hand, calcium can decrease synthesis of cyclic GMP by inhibiting soluble guanylyl cyclase and increase cyclic GMP degradation by activating a calcium-dependent phosphodiesterase (PDE1).
PKG
Protein kinase G enzyme that phosphorylate several proteins after activation by cyclic GMP, act on different steps of the pathway, altering cyclic GMP synthesis and degradation.
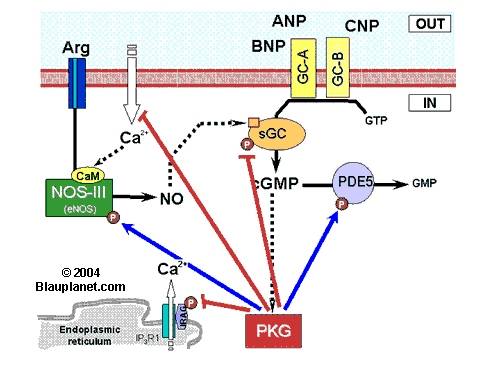
Fig. 1. Hypothetical center role of protein kinase G in pathway regulation. Blue lines represent stimulation of target activity (not an increase in target phosphorylation). Red lines represent decrease of target activity.
Abbreviations:
ANP, atrial natriuretic peptide; Arg, L-arginine; BNP, brain natriuretic peptide; CaM, calmodulin; cGMP, cyclic GMP; CNP, natriuretic peptide C; GC-A (GC-B), particulate guanylyl cyclase type A (or type B), the guanylyl cyclase stimulated by natriuretic petides (ANP and BNP bind to GC-A, while CNP binds to GC-B); Gi, protein Gi; IRAG, Inositol-3-phosphate receptor associated cyclic GMP kinase substrate; NOS, nitric oxide synthase (I, neuronal NOS or nNOS; II, inducible NOS or iNOS; III, eNOS or endothelial NOS);P, represent a phosphorylated aminoacid that results from cyclic GMP-dependent protein kinase activity; PDE5, cyclic nucleotide phosphodiesterase type 5 (it degrades cGMP; this isoenzyme is considered specific for cGMP, however other types of PDE are also able to degrade cGMP); PKG, cyclic GMP-dependent protein kinase; sGC, soluble guanylyl cyclase (guanylyl cyclase stimulated by NO and CO).
The scheme shown is focused on the hypothetical central role of PKG in the regulation of the pathway. PKG directly stimulates NOS-III (or eNOS), sGC itself and inhibits PDE5 (that selectively degrades cyclic GMP). That is, cyclic GMP, through activation of PKG, can modulate its own synthesis (NOS-III and sGC) and its degradation. On the other hand, PKG can also modulate the intracellular calcium concentration, key ion for the activity of NOS-III (eNOS) and NOS-I (nNOS).
Cytosolic calcium is modulated by PKG through several mechanisms that are not specified for simplicity (besides acting on IRAG -shown in the scheme- and on calcium-ATPase at the endoplasmic reticulum, PKG modulates calcium entry at the plasmatic membrane acting at least on L-type calcium channels, on store-operated calcium entry and on mechanosensitive calcium-permeable channels).
Selected references (open access)
Kwan HY, Huang Y, Yao X. Store-operated calcium entry in vascular endothelial cells is inhibited by cGMP via a protein kinase G-dependent mechanism. J Biol Chem 275:6758 (2000). [Full Text]
Mullershausen F, Russwurm M, Thompson WJ et al. Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzyme. J Cell Biol 155:271 (2001). [Full Text]
Rybalkin SD, Rybalkina IG, Feil R et al. Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem 277:3310 (2002). [Full Text]
Ferrero R, Torres M. Prolonged exposure of chromaffin cells to nitric oxide down-regulates the activity of soluble guanylyl cyclase and corresponding mRNA and protein levels. BMC Biochem 3:26 (2002). [Full Text]


Author: Dr. Luis Agulló (luis.agullo [at] lagullo.com).
Edited by Blauplanet.com, Cerdanyola del Vallès, Barcelona, Spain.
Webmaster: info [at] blauplanet.com. Conditions of use.